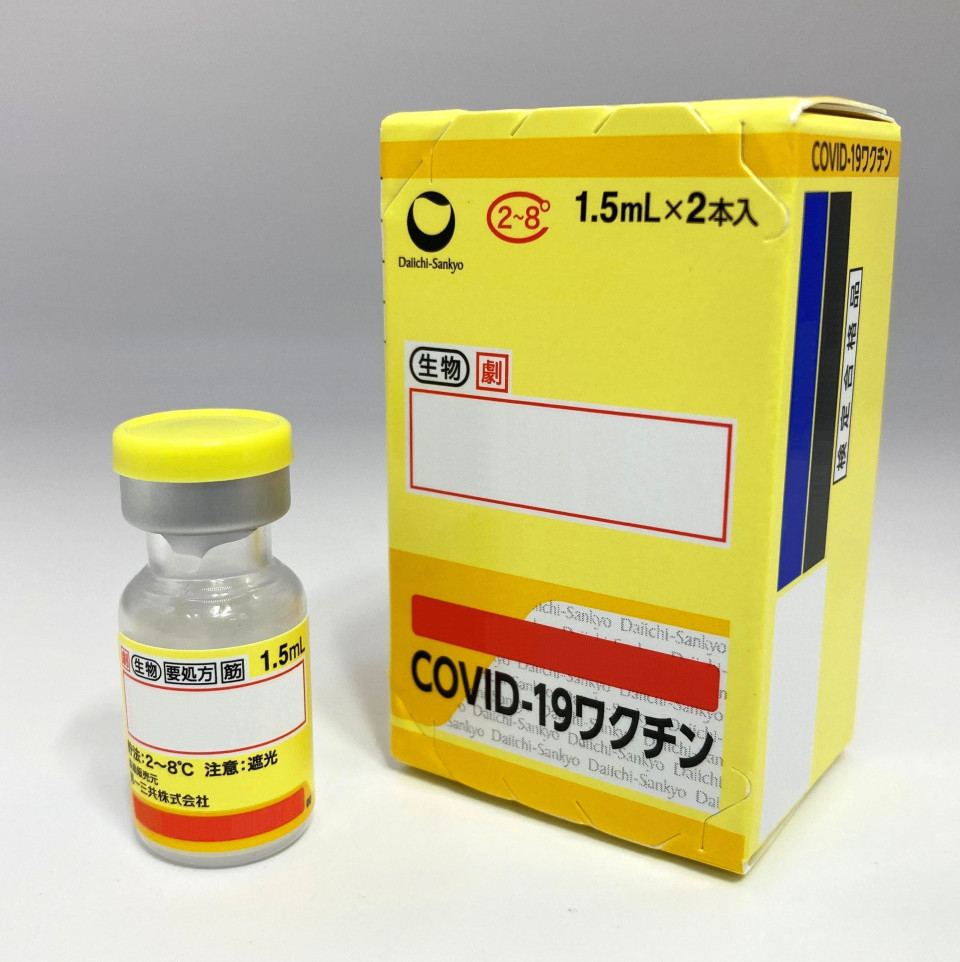
Japan is set to approve the first domestically developed coronavirus vaccine after a health ministry panel of experts on Monday endorsed Daiichi Sankyo Co.'s plan to manufacture and sell the drug.
The Ministry of Health, Labor and Welfare is expected to formally approve the messenger RNA vaccine "Daichirona" soon. The panel, meanwhile, decided to continue the assessment of an application from another Japanese drug maker, Shionogi & Co.
The vaccines developed by both companies are tailored for strains that spread during the initial stages of the pandemic.
In January, Daiichi Sankyo applied with the ministry to approve its vaccine, intended for third-jab inoculations.
In Japan, the messenger RNA vaccines developed by Pfizer Inc. and Moderna Inc., both of the United States, have been in use.
Last November, Shionogi applied with the ministry for approval of its recombinant protein-based preventive vaccine for first and second jabs.
The ministry also plans to introduce from September vaccines that respond to the highly contagious XBB Omicron subvariant. Japan has agreed to purchase the vaccines from Pfizer and Moderna.